Traceability and Compliance with Global Regulations in Medical Device Manufacturing with MES
In medical device manufacturing, enhancing traceability means ensuring that every device and its components can be tracked throughout their lifecycle—from raw materials to finished goods. A centralized traceability system is essential to provide a single source of truth for all manufacturing data within tight timeframes. It supports critical scenarios like audits, product recalls, and faulty supplier batches with strict global regulatory standards.
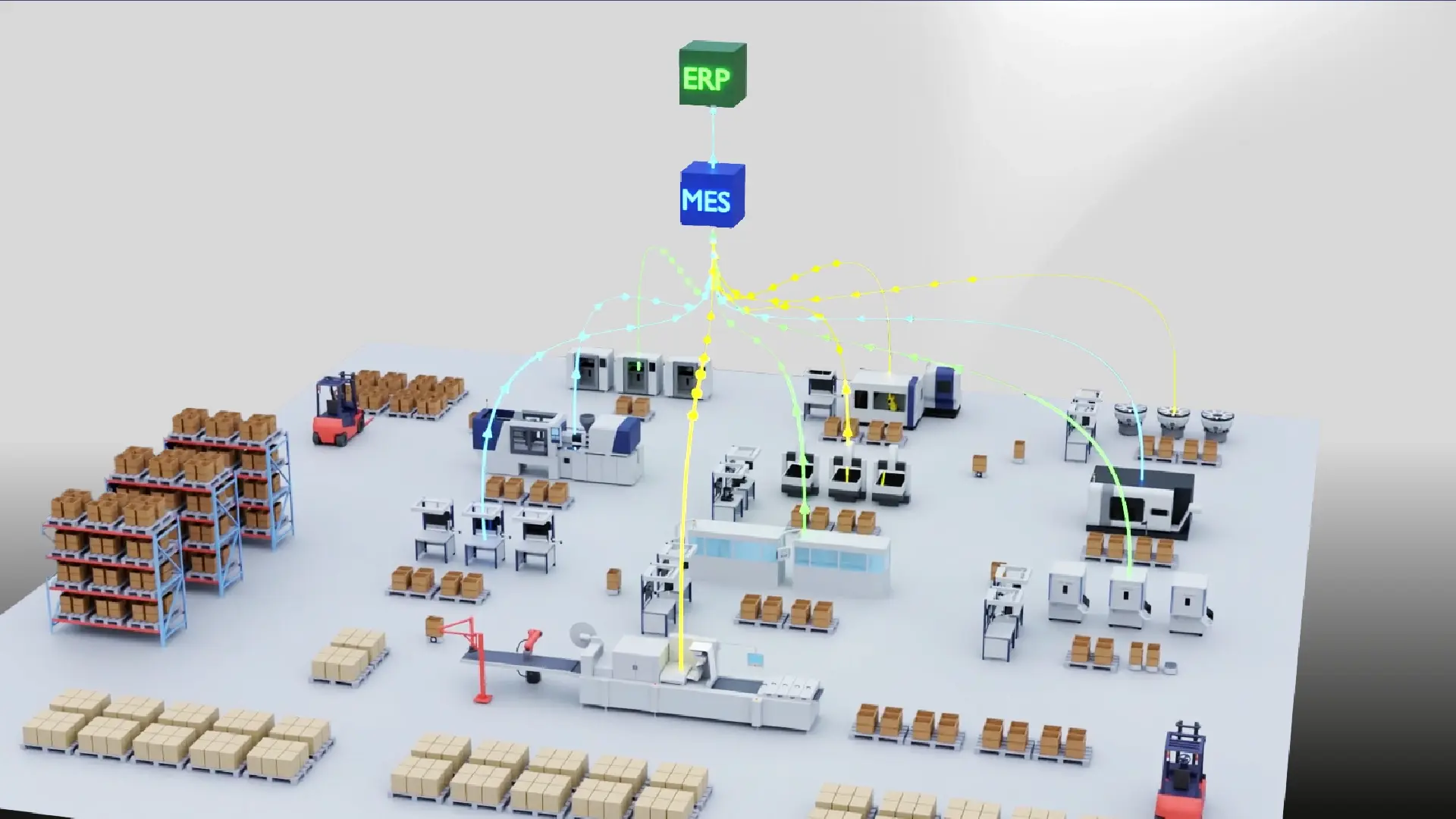
In this Work Smarter video, camLine introduces a powerful all-in-one Manufacturing Execution System (MES) that helps manufacturers transition from paper-based processes to digital excellence. The solution ensures end-to-end data traceability, security, and integrity for material documentation. It also offers seamless integration with your Enterprise Resource Planning (ERP) system and shop floor operations.
Traceability & Compliance in Medical Devices Manufacturing with MES
In medical device manufacturing, enhancing traceability means ensuring that every device and its components can be tracked throughout their lifecycle—from raw materials to finished goods. A centralized traceability system is essential to provide a single source of truth for all manufacturing data within tight timeframes. It supports critical scenarios like audits, product recalls, and faulty supplier batches with strict global regulatory standards.
In this Work Smarter video, camLine introduces a powerful all-in-one Manufacturing Execution System (MES) that helps manufacturers transition from paper-based processes to digital excellence. The solution ensures end-to-end data traceability, security, and integrity for material documentation. It also offers seamless integration with your Enterprise Resource Planning (ERP) system and shop floor operations.
Are Manual Documentation Methods Holding Back Your Medical Device Manufacturing Efficiency?
Many medical device manufacturers still rely on paper-based records or spreadsheets to document and manage production data. While this may be common practice, manual methods often pose the risk of incomplete acquisition of process, machine, and measurement data, as well as difficulties in data harmonization. During audits, product recalls, or regulatory reporting, the absence of data transparency can further complicate decision-making. Learn more about the challenges in medical device manufacturing.
Key Challenges When Relying on Manual Documentation
- Difficulty capturing real-time machine and process data from the shop floor.
- Greater potential for human error and undocumented process variations.
- Slow access to complete production history during audits or inspections.
- Time-consuming and error-prone recall tracing due to fragmented data sources.
- Lack of end-to-end traceability needed to meet global regulatory demands.
Achieve Centralized Traceability and Seamless Integration with InFrame Synapse MES
When production data is stored in separate systems—or worse, managed manually—it becomes difficult to achieve full traceability across your manufacturing processes. For medical device manufacturers, this can result in delays when identifying affected batches, responding to recalls, or preparing for audits. A centralized system with intuitive access to key data points, such as material used, supplier and customer information, and manufacturing timeline, helps eliminate these delays and risks.
InFrame Synapse MES addresses these challenges by enhancing traceability, enabling real-time insights into specific affected products, and allowing the querying of other products with similar manufacturing errors. On top of that, it ensures complete data security and integrity by adhering to ALCOA++ principles, while supporting seamless integration with your ERP and shop floor systems.
How InFrame Synapse MES Benefits Manufacturers
Implementing InFrame Synapse MES empowers medical devices manufacturers by:
- Enhancing Data Traceability: Providing a centralized platform for easy access to critical manufacturing data.
- Streamlining System Integration: Seamlessly integrating with existing systems and ERP to simplify shop floor operations.
- Improving Data Security & Integrity: Utilizing ALCOA++ principles and checksum methods to ensure data accuracy.
- Adhering to Compliance: Complying with strict global regulatory standards from start to finish in manufacturing.
Subscribe to our newsletter
Get our latest updates and news directly into your inbox. No spam.